Research advances in enzyme immobilization and its biosensing applications from Prof. Gangfeng Ouyang’s group at School of Chemistry
Enzymes are the crucial components capable of efficiently catalyzing life-sustaining biological transformations in the complex natural networks, far exceeding artificial catalysts in their efficiency, specificity and selectivity. Nowadays, increasing efforts have devoted to apply enzymatic biocatalysis in sustainable synthesis, drug design, bioengineering, and sensing, etc. Yet, the inherent fragile nature of the enzymes, such as extremely susceptible to temperature and pH fluctuations, and low tolerances to most organic solvents and small molecule inhibitors, etc., significantly restricts the effectiveness of the enzymatic systems in practical operations. Metal–organic frameworks (MOFs) are a class of porous materials made by linking inorganic and organic units with strong bonds (reticular synthesis), with the merits of high surface area, tailorable pore channel, versatile framework compositions and easy-to-surface functionalization, etc. Encapsulating the enzyme in situ within the MOFs cavity (denoted as enzyme@MOFs) is like putting a strong "MOFs armor" on the fragile enzyme, and increasing evidences have been demonstrated that the porous MOFs exoskeletons can not only improve the stability of the encapsulated enzymes through the structural confinement, but also allow the selective mass transfer of the guest via the accessible microporous network of the MOFs exoskeleton. Such symbiotic reinforcement effect between MOFs and enzymes endowed MOFs-embedded enzymes with great potential in sophisticated applications, including biosensing, biocatalysis and bioengineering. However, these initial studies mainly focused on embedding the functional enzymes in the protective MOFs cage without emphasis on the embedding patterns, and limited consideration has been given to the conversion of the enzymatic biofunctionality after entrapment by a MOFs.
Recently, Prof. Gangfeng Ouyang’s group at the School of Chemistry, Sun Yat-sen University, reveal how the embedding patterns influence the biofunctionality of enzymes encapsulated within a MOFs, ZIF-8. Six widely used enzymes in industrial engineering, including glucose oxidase (GOx), cytochrome C (Cyt C), horseradish peroxidase (HRP), catalase (CAT), urate oxidase (UOx) and alcohol dehydrogenase (ADH) were chosen as the enzymatic models, and theirs bioactive conversions after in situ encapsulation within ZIF-8 was studied. The results showed that some enzymes could maintain high biological activity, while other enzymes were partial even completely inactive. In addition, systematic characterizations revealed that the enzymatic activity conversion was closely related to its encapsulation mode: 1) the ZIF-8-embedded enzymes, wherein the enzymes were encapsulated through the rapid self-triggered nucleation around their surface, could maintain high enzymatic activity; 2) the encapsulation was driven by the slow coprecipitation procedure wherein the enzymes were not involved in the nucleation of ZIF-8, the obtained enzymes@MOFs tended to be inactive owing to the unfolding effect and competing coordination caused by the ligand, 2-methyl imidazole (Figure 1A). Interestingly, these two embedding patterns were depended on the surface chemistry of the enzymes, and the researchers demonstrated that the embedding patterns were facile to be controlled via chemical modification of the amino acids of the enzymes that enables to modulate the biofunctionality of the encapsulated enzymes. Finally, the potential of optimized enzymes@MOFs for bioanalysis was explored. Firstly, the single enzyme@ZIF-8, including Cyt C-A@ZIF-8 and HRP-A@ZIF-8 was applied for visual H2O2 sensing (Figure 1B). Given the sensitive H2O2-sensing performance of HRP-A@ZIF-8, a new platform for biothiols sensing was also established. Glutathione (GSH) is one kind of biothiols that has a variety of physiological functions related to diabetes, liver disease, cataracts and Alzheimer's and Parkinson's disease. GSH is facile to be oxidized by H2O2, and thus can inhibit the catalytic efficiency of HRP-A@ZIF-8. Such catalytic inhibition effect could be utilized for visual GSH sensing, with high sensitivity and wide linear range (Figure 1C). Compared with single-enzyme catalysis, multi-enzyme catalytic cascades represent a major class of chemical transformations that play key functions in biological signal transduction and metabolic pathways in the cellular environment. The researchers co-encapsulated GOx and HRP within MOFs (ECMN, Figure 1D) to simulate intracellular cascade catalysis. Through controlling the embedment patterns, the bioactivity of catalytic cascades of ECMN was improved (Figure 1E), and the constructed ECMN was utilized as the platform for visual and sensitive glucose sensing (Figure 1F).

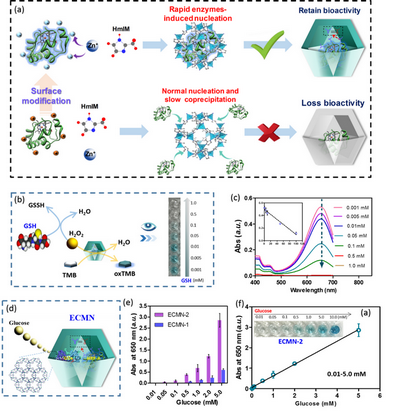
Figure 1. (A) Schematic representation of the rapid enzymes-triggered nucleation and slow coprecipitation embedding patterns; (B) Schematic representation of visual GSH sensing by HRP-A@ZIF-8; (C) Dependence of the intensity of the absorption intensities at 650 nm on the concentration of the GSH;(D) Schematic representation of the enzyme cascade MOFs nanoreactors, ECMN; (E) The improvement of biocatalytic cascade in ECMN. (F) Sensitive and visual detection of glucose.
This work was published in Angewandte Chemie International Edition (IF=12.257), a renowned chemistry journal in the world. The first author is Dr. Guosheng Chen, and the corresponding author is Prof. Gangfeng Ouyang. Full text links: https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201913231.
In addition, Dr. Guosheng Chen and Prof. Gangfeng Ouyang’s group reviewed the advanced progress of enzymes@MOFs in terms of encapsulation strategies and cutting-edge applications, with special emphasis on the pore size of MOFs for enzymes packaging, biointerface between the enzymes and metal node that facilitating in situ encapsulation and the key factors influencing the enzymatic activity conversion. Furthermore, the cutting-edge applications of enzymes@MOFs in biocatalysis, sensing and nanocatalysis therapeutics, etc. were also introduced. The relevant work, entitled "Armoring the Enzymes with Metal - Organic Framework", was published in the chemical comprehensive journal Angewandte Chemie International Edition. The first author is Dr. Siming Huang, a postdoctoral fellow at Sun Yat-sen Memorial Hospital, and the corresponding author is Dr. Guosheng Chen and Prof. Gangfeng Ouyang. Full text links: https://onlinelibrary.wiley.com/doi/10.1002/anie.201916474.
These works were financially supported by projects of the National Natural Science Foundation of China (21904146, 21737006, 21677182, 21527813 and 21477166), postdoctoral Innovative Talents Support Program (Boxin Plan, BX20190390), China Postdoctoral Science Foundation (2019M653138) , Natural Science Foundation of Guangdong Province (2019A1515011722), and Fundamental Research Funds for the Central Universities (19lgpy125).



